
Organophosphates are widely used in horticulture as insecticides, fungicides, ascaricides and soil fumigants. In agriculture, these compounds are applied to animals as dips, sprays, pour on preparations, drenches, powders and in flea collars. The preference for these compounds to be used was their distinct advantage over the previously used chlorinated hydrocarbons because they do not accumulate in animal and plant tissues and are rapidly hydrolysed and degraded both in the animal and the environment. Organophosphate compounds have also been used as plasticisers, stabilisers in lubricating and hydraulic oils, flame retardants, gasoline additives and anthelmintics.
Some of the organophosphorus compounds used in horticulture and agriculture in New Zealand. Many of these are now unpopular or no longer licensed for use. Some have been replaced by more efficient and safer preparations.
| Chemical name | Trade names | Use |
|---|---|---|
| Chlorfenvinphos | Flystrike Dressing | Insecticide |
| Dichlorfenvinphos | Supreme | |
| Chlorpyrifos | Flypel | Animal and environmental pesticides |
| Coumaphos | Asuntol(Bayer) | Animal insecticide, miticide. Insecticide cattle and pigs. |
| Cythioate | Cyflee | Ectoparasiticide |
| Diazinon | Basudin, Gesapon | Most widely used OP insecticide dips and flea collars etc. |
| Dichlorvos | De de Vap, Equiminth Plus, Nuvan etc | Insecticide and equine anthelmintic |
| Famphur | Warbex | Lice control in cattle |
| Fenitrothion | Caterkil, Verthion | Insecticide |
| Fenthion | Lucijet Bayer, Tiguvon, Spotton, Trigon | Fly control and other ectoparasites |
| Maldison | Parasite Spray | Insecticide |
| Phosmet | Porect, Paramite, Poron 10 | Non systemic insecticide and ascaraside |
| Propetamphos | Destruct, Seraphos | Non systemic insecticide |
| Temephos | Lypor Cattle, Tempor | Lice, midge and mosquito control |
| Trichlorphon | Neguvon | Non systemic and systemic insecticide and parasiticide |
Organophosphorus insecticides are organic trimesters of phosphoric, thiophosphoric or dithiophosphoric acids. The toxicity of the compounds depends on the nature of the so called acyl radical, which determines the ease with which esterases such as acetylcholinesterase can hydrolyse the molecule and become phosphorylated.
The toxicity factors of organophosphates are multiple and usually poorly understood. Many pesticides are degraded by the sun, water, microorganisms and in the presence of either alkyl groups or metal ions, e.g. iron and copper. However, a number of organophosphates undergo storage activation in which highly toxic isomers of certain pesticides are formed spontaneously in polar solvents or water. Compounds which undergo storage activation are parathion, malathion, fenitrothion, fenthion, abate, dimethoate, chloropyrifos, diazinon, carbophenothion, ethion, coumaphos, demeton, methyl demeton. It is best to use only freshly prepared suspensions, emulsions or solutions.
The susceptibility to acute organophosphate toxicity varies with the species and breed because of genetic differences in acetyl cholinesterase levels. Cats are the most susceptible species, followed by sheep and cattle, dogs, pigs and horses. Even within species, certain breeds e.g. Brahman cattle, exhibit considerable individual variation in susceptibility to organophosphates.
The sex of the animal, also has a marked influence on toxicity. Brahman bulls are more susceptible than Brahman heifers to dioxathion. Female rats are more susceptible than male rats to organophosphates which are degraded by liver microsomal enzymes, and male rats are more susceptible than female rats to organophosphate substances which are activated by liver microsomal enzymes.
The concentration of the liver enzymes is affected by the age of the animal. Young animals are more susceptible to OP compounds which do not require hepatic enzyme activation. However, organophosphates which do require hepatic metabolism are less toxic to the same group of animals.
Nutritional stress, infection or toxic factors of other types (e.g. plant poisoning), may make animals treated with OP compounds susceptible to normal therapeutic doses. Concurrent therapy with another OP can have synergistic or antagonistic effects, as can any drugs which have neuromuscular blocking properties. These drugs include inhalant anaesthetics, magnesium ions, certain antibiotics e.g. streptomycin, dihydrostreptomycin, neomycin, kanamycin, gentamycin, polymixin B, and depolarizing and nondepolarizing blocking agents. Any pesticide (e.g. chlorinated hydrocarbons) with an action similar to organophosphates may have synergistic effects, and agents capable of inducing hepatic enzymes (e.g. phenobarbitone, chlorinated hydrocarbons, pesticides, polychlorinated biphenyls and others), may increase, reduce or not alter the toxicity depending on the OP, the species and age of the animal.
Organophosphorus compounds are poorly soluble in water and as such are widely used as dusts, wettable powders and emulsions. These compounds are soluble in organic solvents, fats and oils and can penetrate into waxy coats of leaves, fruit and can be directly absorbed through the skin of humans and animals.
The organophosphate insecticides are readily absorbed through all routes (e.g. oral, dermal and respiratory) although orally administered OPs are the most toxic. The rate of dermal absorption is considerably affected by the solvent used. Organophosphates are rapidly distributed around the body and do not accumulate in any particular tissue. Most compounds are not potent inhibitors of acetylcholinesterases, until they are activated in the liver, primarily by microsomal oxidative enzymes. Organophosphate compounds which do not require activation include: dichlorvos, naled, propophos, cyanthioate, chlorfenvinphos, and tetrachlorvinphos.
Most organophosphates intended for animal use are rapidly excreted with minimal tissue or blood residues within a few hours of exposure. Residues will vary with the compound, dose and route of exposure.
The best known toxic effect of organophosphates is the direct inhibition of acetylcholinesterase, which is the enzyme responsible for the hydrolysis of acetylcholine. This causes acetylcholine to accumulate beyond normal levels, which causes continuous stimulation of the effector cells and muscle fibres.
Two main types of cholinesterase enzymes are recognised in most animals. One is true cholinesterase which has a specificity for acetylcholine and is found in the nervous system, muscles, glands and erythrocytes. Inhibition of this enzyme type is most important in the production of organophosphorus toxicity. A second non specific or pseudocholinesterase is found in plasma and other tissues, including the nervous system and is capable of inactivating various esters, including acetylcholine. The cholinesterase found in whole blood is generally not directly involved in the appearance of signs of poisoning due to cholinesterase depletion in the nervous system. Hence on occasion animals will show signs of organophosphorus poisoning with only a slight depression of blood cholinesterase or the reverse situation can occur. In general however depletion of blood cholinesterase does correlate with the clinical signs shown.
The result of cholinesterase depression is to produce three broad categories of clinical signs referable to (1) the muscarinic receptors for acetylcholine found in smooth muscle, (2) nicotinic signs due to acetylcholine at motor nerve endings and autonomic ganglia and (3) central nervous system signs.
The sequence of events in organophosphorus poisoning can be schematically summarised as follows.
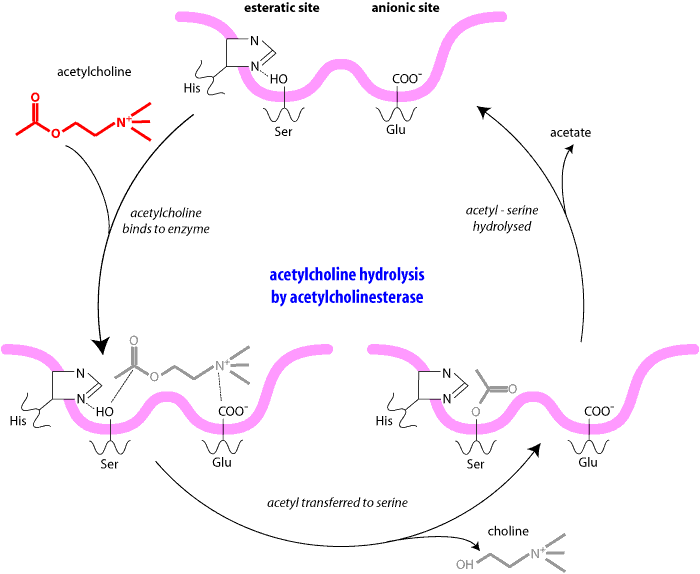
The physiological action of cholinesterases on their normal substrate acetylcholine takes place in three stages.
Most organophosphates are not very persistent in the environment and dissipate within two to four weeks. Fensulfothion, prophos and trichloronate may persist in the soil for more than four weeks, and chlorfenvinphos, phospholon, dichlorfenthion and oxydisulfon may persist in the soil for more than thirty six weeks.i.e. the presence of a phosphoryl group on the enzyme (at Stage I) blocks the access of acetylcholine molecules preventing the hydrolysis of the substrate and causing acetylcholine to accumulate.
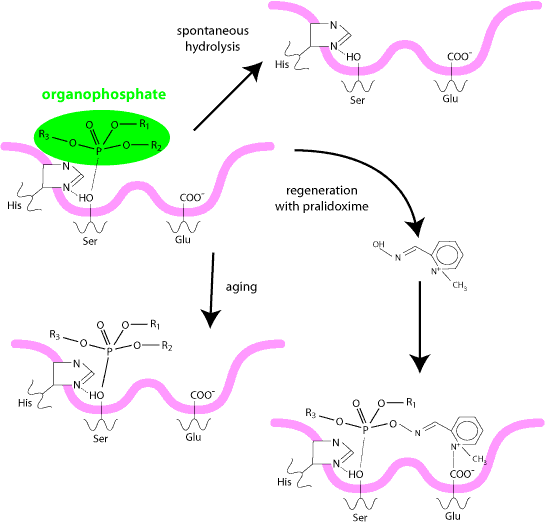
In summary, a phosphorylated enzyme rather than an acetylated enzyme is formed and this is considerably more stable and cannot readily be hydrolysed, so that no regeneration of cholinesterase is able to take place and acetyl choline accumulates.
Certain organophosphate compounds bind neurotoxic esterase in the brain and spinal cord, forming a complex that ages and destroys nerve axons and myelin sheaths. This results in neurotoxicities which show symptoms months to years after exposure to the organophosphate compound.
Most organophosphates are metabolised to water soluble metabolites which are then excreted through the kidneys. Many of the OP's are excreted in the milk, but under normal circumstances will only be present in the milk for up to 7 days. Concentrations in the milk can reach toxic levels in sows following topical treatment with diazinon; resulting in deaths of piglets.
Some organophosphate compounds e.g. parathion, methyl parathion, malathion, diazinon and dichlorvos are known to cross the placenta in goats and rats, to produce depressed foetal cholinesterase levels, low foetal weight and foetal abnormalities, e.g. cleft plate, foetal death, post natal death, hydronephrosis, hydroureter and abortion.
The signs of acute organophosphorus poisoning are predictable by virtue of the known biochemical mechanism of action. Accumulation of acetyl choline leads to overstimulation of the parasympathetic nervous system and of the post ganglionic nerves of the sympathetic system. Essentially three types of signs are seen.
Initially animals may emanate an insecticide or garlic odour from the coat or breath, but the first clinical signs are usually due to the stimulation of the muscarinic receptors resulting in:
Muscarinic effects
SLUDDE is an acronym used to recall the main clinical signs:
Slightly later or almost simultaneously the nicotinic effects may show. These can be particularly spectacular in some cases.
Nicotinic effects
Other nervous signs are due to the effect on the central nervous system and include:
Central effects
Death is usually due to respiratory failure and cardiac arrest.
In general there is little difference in the symptoms produced by different compounds but depending on the route of absorption some systems may be more affected than others e.g. in cases of inhalation exposure to O/P compounds, the respiratory system may be more severely affected than the digestive system.
Signs may appear within minutes of inhalation or ingestion of toxic O/P compounds and deaths may occur within 2 - 5 minutes later. With less toxic compounds there may be a delay of several hours before signs appear and deaths may occur 12 - 24 hours later. Some organophosphates can cause a myopathy due to skeletal muscle necrosis and diazinon, dichlorvos, parathion and others have a teratogenic effect. Abortion may occur, particularly during the last third of pregnancy.
Organophosphorous poisoning may show localised effects in the absence of systemic effects. Exposure to dust, vapours and aerosols can cause local irritation to the eyes and respiratory tract. Local dermal exposure may cause localised sweating or muscular fasciculations at the site of contact.
Continuous exposure to OP compounds may lead to the development of an apparent adaption of the acetylcholinesterase to high local acetylcholine, which further complicates the significance of the depressed or zero red cell acetylcholinesterase levels. Chronic exposure to small amounts of organophosphate compounds produces the development of tolerance, due to the induction of hepatic microsomal oxidative enzymes, the continual synthesis of esterases, the physiological adaption to reduced esterase levels and the adaption of cholinergic receptors to the presence of excessive amounts of acetylcholine.
Delayed neurotoxicity has been reported in horses and cattle in New Zealand and no doubt seen in other species. In one case described which affected eight yearling cattle, severe signs of neurotoxicity were very evident at 28 days. Some died but others still displayed these signs up to a year later.
Organophosphorous compounds can phosphorylate esterases rather than acetylcholine esterase and in particular esterase in nerve cells may be inactivated. This may result in a sensory and motor peripheral neuropathy. The enzyme has been referred to as "neurotoxic esterase". The effect is felt most severely in neurons of large diameter and long axons such as those which innervate the hind limbs. The sensory nerves are earliest and most severely affected but motor nerves are affected as well.
The earliest observed effects are disturbances in proprioception, leading to ataxia and posterior paralysis, particularly in exercise. In severely affected animals the forelimbs may also be involved. The lesion appears to be a metabolic disturbance of the axon leading to its slow centripetal degeneration or "dying back".
"Dying back" of the axon is accompanied by typical Wallerian degeneration, the lesions of which are most readily seen in the cervical cord. A peculiar aspect of the degeneration is that no matter how high the dose of OP compound, the ataxia never appears before 8 days of dosing. It tends to increase in intensity for some 3 weeks after which regeneration may slowly take place. This phenomenon has been reported in man, cattle, horses, sheep, pigs, dogs and poultry. In humans the disease has been recognised for several decades and was earlier referred to as Ginger Jake paralysis. Tri orthocresyl phosphate (TOCP) was the contaminant OP found in one case in an alcoholic beverage and in another as a contaminant of cooking oil.
There are no characteristic necropsy lesions associated with organophosphate intoxication, although intoxicated animals often emanate a garlic odour. Gross changes are usually associated with a haemorrhagic gastroenteritis, pulmonary oedema, with excessive upper respiratory tract secretions, but these changes are usually secondary to the clinical signs. In delayed neurotoxicity, histopathology reveals degenerative lesions in the large peripheral nerves of the spinal cord and medulla.
The history of access and clinical signs usually suggest organophosphate poisoning. A definite diagnosis can be made by analysis of blood and tissue samples.
The test for organophosphates is expensive and tedious. The best samples to send are blood, vomit, stomach contents, fresh liver and fat although often the results are negative, as organophosphate compounds are not stored in tissues, but are rapidly broken down by the liver. Urine may contain metabolites. If the exposure was dermal then skin or hair may be submitted. Detection of more than a trace of OP in the tissue is significant, when associated with characteristic signs.
The best indication of intoxication is the blood acetylcholinesterase concentration. Local diagnostic laboratories require a serum blood sample from monogastrics and an EDTA sample from ruminants to be submitted with that from a normal animal. The acetylcholinesterase level is usually less than 50% of the control animal, if there has been exposure to an anticholinesterase agent.
The determined concentration varies markedly depending on the reagent used, whether it is measured on blood or plasma (some laboratories recommend heparinised blood) and the temperature of the reaction should be stipulated.
Many organophosphate intoxicated animals show respiratory distress and cyanosis, so the initial treatment should aim to establish a patent airway, and provide oxygen, including artificial respiration if necessary.
Atropine is a muscarinic antagonist and will reduce the muscarinic effects, but has no influence on the nicotinic like signs, hence the animal will continue to shiver, twitch or remain paralysed.
Dose in very sick animals give one third of dose iv and the rest im or sc. Sheep can tolerate larger doses of atropine than can cattle.
*Some rabbits have atropinase and may require higher doses of atropine. Start with 1 mg/kg and increase as needed.
Use caution in horses (0.1 - 0.2 mg/kg). If necessary to use atropine monitor gastrointestinal sounds and stop if GI motility decreases.
In all species repeat treatments 4 - 5 hourly for up to 48 hours until improvement is seen. It should also be noted that animals poisoned with OP compounds are relatively resistant to atropine and doses much higher than normal may be given with safety. Atropine should bring about relief from the acute signs very quickly.
A light degree of atropinization exists when the pupils are dilated, salivation ceases and the animal appears to be recovering.
The second part of the specific therapy involves using some compound which will reactivate the phosphorylated cholinesterase. Pralidoxime (pyridine 2 aldoxime methiodide, 2 PAM) causes an effective removal of the phosphate group from the enzyme, so that the enzyme is reactivated.
Dose twice a day as required generally until nicotinic signs resolve
Atropine and pralidoxime give rapid relief provided the animal has been exposed within a few hours, as the phosphorylated complex ages with time and becomes refractory to treatment.
Administration of pralidoxime without prior treatment with atropine has only minimal effect, as atropine is required to block the muscarinic enzymes sites. The quantity of atropine required is greatly reduced and the recovery is much quicker. Pralidoxime is of more value in some OP compounds than others but is said to be ineffective with dimethoate and schroden. The best known pralidoxime compound is a chloride salt, but the iodide or mesylate salts are also used. Various other reactivation oximes - pyridine 2 aldoxime; dodecaiodate, monoisonitroacetone and diacetylmonoxime have also been investigated, but pralidoxime is still the compound of choice. The use of oxime compounds is controversial in the treatment of carbamate intoxication, it is thought the two drugs may act synergistically. If in doubt whether it is a carbamate or organophosphate compound, but clinical signs suggest a cholinesterase inhibitor use an oxime such as pralidoxime.
Removal of any residual organophosphate compounds is important, if the animal is not to relapse. This involves washing the animal with water and alkaline soap, if dermal toxicity is suspected or gastric lavage and administration of activated charcoal, if the route was oral. Mineral oil should be avoided, as the oil may increase the absorption of the poison from the gastrointestinal tract.
Diphenhydramine, an antihistamine, has been shown to have beneficial effects in the treatment of organophosphate intoxication by reducing the clinical signs of excessive nicotinic receptor stimulation. Diphenhydramine has been proposed in the treatment of organophosphate induced muscular weakness which is refractory to other forms of therapy. It should not be administered in conjunction with atropine.
Dose Dogs 4 mg/kg
The animal may require several days to recover, and the appropriate supportive treatment - fluids, electrolytes and multiple vitamins should be administered.
There is no antidote to the neurotoxicity. The animal may survive if it is given intensive nursing care, although the muscular weakness and prostration may last for weeks. If the pesticide is one which causes degeneration and demyelination of peripheral and spinal motor neurones then euthanasia may be indicated. It is important to determine whether the muscular weakness is a direct neurotoxic reaction or prolonged weakness from the anticholinesterase activity of slowly absorbed or slowly activated pesticide, or incomplete dephosphorylation of acetylcholine by oximes. As long as the animal is eating and drinking, it is worthwhile waiting for several days even to weeks for recovery.
During the acute or recovery phase of organophosphate intoxication, central nervous depressants (e.g. phenothiazine, barbiturates, succinylcholine, tranquillizers and opiates) must be avoided. Diazepam may be indicated for the treatment of convulsions.
Adams, H.R. (1982). Cholinergic pharmacology:Autonomic drugs.In Veterinary Pharmacology and Therapeutics.Ed. Booth, N.H. and McDonald, L.E.The Iowa State University Press/Ames. 123 126.
Andrews, A.H. (1981). Abnormal reactions and their frequency in cattle following the use of organophosphorus warble fly dressing. Vet Rec. 109: 171 175.
Barrett, D.S., Oehme, F.W. and Kruckenberg, S.M. (1985). A review of organophosphorus ester induced delayed neurotoxicity. Vet Hum Toxicol. 27: 22 37.
Clemmons, R.M., Meyer, D.J., Sundof, S.F., Rappaport, J.J., Fossler, M.E., Hubbell, J. and Dorsey Lee, M.R. (1984). Correction of organophosphate induced neuromuscular blockade by diphenhydramine. Am J Vet Res 45:2167 2169.
Cook, T.F. (1966). Toxicity of Haloxon in sheep. N Z vet J. 14:71 72.
Harrison, D.L. and Hastie, B.A. (1965). Diazinon residues in the milk of cows and fat of sheep after feeding on pasture treated with diazinon. N Z J Agric Res. 9:1 7.
Hatch, R.C. (1982). Poisons causing nervous stimulating or depression. In Veterinary Pharmacology and Therapeutics. Ed. Booth, N.H. and McDonald, L.E.The Iowa State University Press/Ames. 984 997.
Jones, J.M. (1984). Organophosphorus poisoning in two Rex rabbits. N Z vet J. 32: 9 10.
Khan, M.A. (1973). Toxicity of systemic insecticides: Toxicological considerations in using organophosphorus insecticides. Vet Rec Reece, R.L. (1982). Observations on the accidental poisoning of birds by organophosphate insecticides and other toxic substances. Vet Rec. 111:453 455.
Solly, S.R.B. (1971). Veterinary aspects of insecticides: Organophosphates. N Z vet J. 19:233 240.
Stuart, L.D. Organophosphorus delayed neurotoxicity: A neuromyelopathy of animals and man. Vet Hum Toxicol 24:107 118.
Thompson, J.C. Thompson, A.H. and Thornton, R.N. (1993). Accidental poisoning of a group of yearling cattle by the organophosphate insecticide trichloronat. N Z vet J. 41:87 90.
Surveillance (1974) 1(3): 2 Severe organophosphorus poisoning of dairy cattle
Surveillance (1975) 2(2): 23 Diazinon poisoning (ducks)
Surveillance (1975) 2(3): 7 Organo phosphate potentiation
Surveillance (1975) 2(5): 16 Fenthion toxicity
Surveillance (1978) 5(1): 8 Phorate poisoning of seagulls
Surveillance (1978) 5(1): 14 Malicious organophosphate poisoning
Surveillance (1979) 6(1): 16 Organophosphate poisoning (dogs)
Surveillance (1979) 6(1): 17 Organophosphate poisoning (dogs)
Surveillance (1979) 6(4): 19 Organophosphorus poisoning (budgies)
Surveillance (1980) 7(1): 10 Poisonous purse snatching (dogs)
Surveillance (1980) 8(1): 16 Diazinon poisoning in newly born pigs
Surveillance (1981) 9(3): 21 Organophosphate poisoning
Surveillance (1981) 9(3): 23 Neuronal storage disease and organophosphate poisoning in a dog
Surveillance (1981) 9(1): 19 Organophosphate poisoning in dogs.
Surveillance (1997) 24(4): 21 Organophosphate poisoning of cattle.
Surveillance (1998) 25(3):17 Organophosphate poisoning of ducks.