
Different from most drugs in that the inhalation agents are taken up through the lungs and excreted (mostly) unchanged by the same route. (Some of the older agents may undergo significant metabolism.)
This is an important concept. It is the concentration in the alveolus at a steady state which will prevent purposeful movement in response to a supramaximal stimulus in 50% of individuals (ie, it is a type of ED50). It is lowered by sedatives, induction agents, analgesics, nitrous oxide. Usually about 1.3 MAC is required for maintenance but the actual amount required will depend on animal’s state of excitment / pain. The MAC is used to compare the potency of inhalation anaesthetics.
Alveolar concentration is used as it is assumed that alveolar
gas has equilibrated with alveolar capillary blood; since very little drug is
likely to be absorbed into other tissues before this blood reaches the brain,
this equates roughly to brain concentration (assuming the drug crosses the blood
brain barrier easily).
Do not read this bit if you are easily confused!
When inhalation anaesthetics were first used, they were administered by pouring some onto a wad of cotton wool and holding this over the animal’s nose. Since the effects of these drugs are critically dose dependant, and overdose results in death, precision vaporisers and complicated anaesthetic circuits were invented to try to control the dose the animal receives. To deliver the correct dose of drug to the animal you must understand how this equipment works (and how to repair it when it doesn’t)!
Modern precision vaporisers are very accurate but very expensive. In human anaesthesia, there is a trend back towards using simple vaporisers (or even just squirting a bit of inhalation agent in the circuit with a glass syringe) and using sophisticated monitoring equipment to check how much drug the patient is getting. This sort of monitoring equipment is also expensive and is not widely used in veterinary practice.
If animal is healthy, alveolar concentration is proportional to brain concentration, but lung disease will slow the uptake of drug. A number of factors influence uptake and elimination:
physical factors (properties of the drug) (table)
• saturated vapour pressure - mainly a factor in vaporiser design unless you use a vaporiser with an anaesthetic for which it was not designed (or a non-precision vaporiser). As vaporisers are very expensive, this is sometimes done but is not recommended. It is useful to express SVP in kPa - this approximates to the maximal concentration you can get out of the vaporiser.
• rubber solubility - mainly a problem with older agents The drug has to pass through lots of rubber / plastic tubing before it gets into the animal.
• blood brain coefficient - not clinically significant with current drugs, they all get into the brain very easily.
• blood gas coefficient (solubility) This determines speed of induction and recovery. A relatively insoluble anaesthetic will quickly reach equilibrium between inspired and alveolar concentration, a relatively soluble anaesthetic will take a long time. A soluble anaesthetic effectively has a larger volume of distribution, so more drug has to be absorbed to fill this volume and this takes longer. Therefore a relatively insoluble agent will give a fast induction and recovery; eg, desflurane (BG coeff 0.4; ie insoluble) will give a faster induction and recovery than ether (BG coeff 12; ie soluble). NB., if a drug is completely insoluble it will not get to the brain and will not be an anaesthetic. Soluble drugs tend to be more potent - anaesthesia can be produced at lower concentrations. If this did not happen, they would not be useful clinically as it can take a very long time for them to reach high concentrations.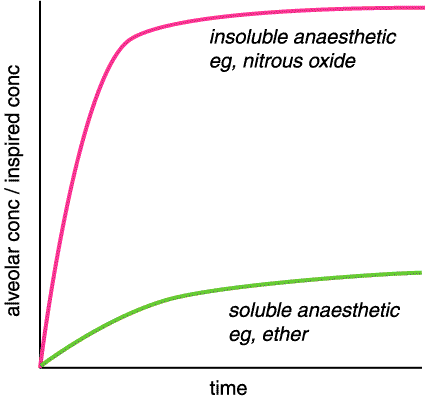
Uptake of inhlation anaesthetic agents. Note that the Y axis is a ratio rather than an absolute concentration.
other factors (properties of the animal)
• ventilation - in the most extreme case, if an animal is not breathing, it will not take up any drug. (Beware, during intermittent positive pressure ventilation large quantities of drug can be forced into the animal.)
• cardiac output - again in the most extreme case, if cardiac output is zero, the blood containing the drug will not leave the lungs and systemic uptake will be zero. A low cardiac output will slow the passage from the lungs to the brain.
• second gas effect- probably only important with nitrous oxide. If a large proportion of the volume of the gas in the alveolus moves across into the blood, more fresh gas will move into the alveolus to take its place, bringing more halothane (or whatever) with it. This is a way of getting more halothane into the animal faster, ie, faster induction. This will only happen for the first few minutes - it only takes about 10 minutes for nitrous oxide to equilibrate. The opposite occurs on recovery (Fink effect or diffusion hypoxia) where the nitrous oxide diffuses from the blood into the alveolus displacing alveolar gas (including oxygen); this can give rise to hypoxia. 100% oxygen is usually given for several minutes on recovery from an anaesthetic which has used nitrous oxide.
• lung disease - thickening of the blood gas barrier in the lungs will slow diffusion. This will mean a slower uptake and elimination of anaesthetic.
Conventions on dosage of inhalation anaesthetics are slightly confusing. The amount of drug the animal is given is usually expressed as a percentage of inspired gas (sometimes the fraction - FI drug). Once in the animal it is sometimes expressed as a partial pressure or tension (particularly for gases). This can lead to confusion as there are at least six different units of pressure used in anaesthesia. Remember that at sea level the ambient pressure is approximately 101kPa (the SI unit), 1 atmosphere, 1 bar, 1000mbar, 760 mmHg, 15 psi or 1000 cm water! Therefore 4% halothane = 0.04 FI hal = 4 kPa
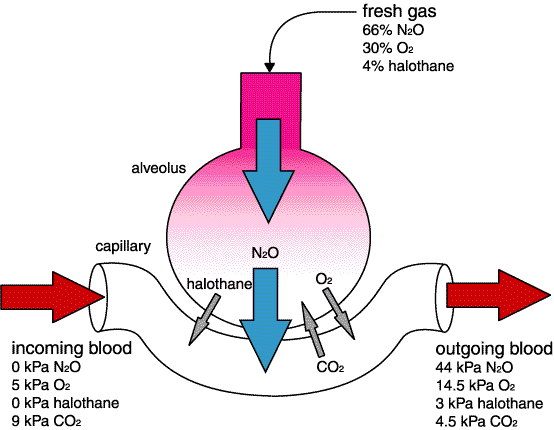
Second gas effect - confusing but not clinically important except when using nitrous oxide.